Researchers at the Lawrence Berkeley National Laboratory have demonstrated a lithium-sulfur (Li/S) battery that has more than twice the specific energy of current lithium-ion batteries, and that lasts for more than 1,500 charge/discharge cycles with minimal capacity loss. This is the longest cycle life yet reported for any lithium-sulfur battery. The results were reported in the journal Nano Letters.
“The lithium-sulfur battery chemistry has attracted attention because it has a much higher theoretical specific energy than lithium-ion batteries do,” says Elton Cairns, one of the paper’s co-authors. “Lithium-sulfur batteries would also be desirable because sulfur is nontoxic, safe and inexpensive.” Li/S batteries would be cheaper than current Li-ion batteries, and they would be less prone to safety problems such as overheating and catching fire.
However, Li/S also has its challenges. During discharge, lithium polysulfides tend to dissolve from the cathode in the electrolytes and react with the lithium anode, forming a barrier layer of Li2S. This chemical degradation is one reason why the cell capacity begins to fade after just a few cycles. Another problem is that the conversion reaction from sulfur to Li2S and back causes the volume of the sulfur electrode to swell and contract up to 76% during cell operation, which leads to mechanical degradation of the electrodes.
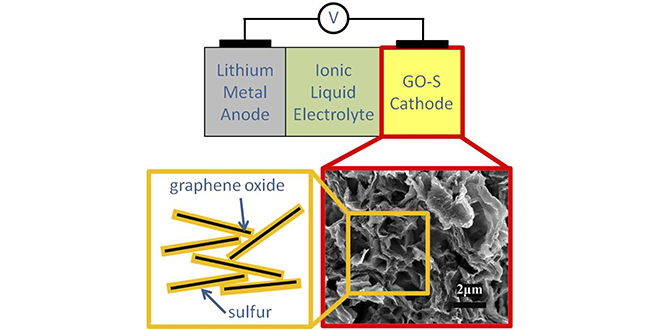
The prototype cell designed by the research team uses several electrochemical technologies to address these problems. The cathode is composed of sulfur-graphene oxide (S-GO), a material developed by the team that can accommodate the volume change as sulfur is converted to Li2S on discharge, and back to elemental sulfur on recharge. To further reduce mechanical degradation, the team used an elastomeric binder. Combining elastomeric styrene butadiene rubber (SBR) binder with a thickening agent enabled substantial improvements in cycle life and power density.
To address the problem of polysulfide dissolution and chemical degradation, the research team applied a coating of cetyltrimethyl ammonium bromide (CTAB) surfactant to the sulfur electrode, which reduces the ability of the electrolyte to penetrate and dissolve the electrode material.
The team also developed a novel ionic liquid-based electrolyte, which inhibits polysulfide dissolution and helps the battery operate at a high rate, increasing the speed at which the battery can be charged, and the power it can deliver during discharge. The ionic liquid-based electrolyte also improves safety, as ionic liquids are non-volatile and non-flammable.
The battery initially showed an estimated cell-specific energy of more than 500 Wh/kg and maintained it at >300 Wh/kg after 1,000 cycles – much higher than that of currently available lithium-ion cells, which currently average about 200 Wh/kg.
“It’s the unique combination of these elements in the cell chemistry and design that has led to a lithium-sulfur cell whose performance has never been achieved in the laboratory before – long life, high rate capability, and high cell-level specific energy,” says Cairns.
An EV with a 300-mile range would require a battery with a cell-level specific energy of 350-400 Wh/kg, almost double the specific energy (about 200 Wh/kg) of current lithium-ion batteries. The battery would also need to endure 1,000-1,500 charge/discharge cycles without substantial power or energy storage capacity loss.
The next steps are to further increase energy density, improve cell performance under extreme conditions, and scale up to larger cells. The research team is now seeking support for continuing development, and partnerships with industry.
Source: Lawrence Berkeley National Laboratory via Green Car Congress